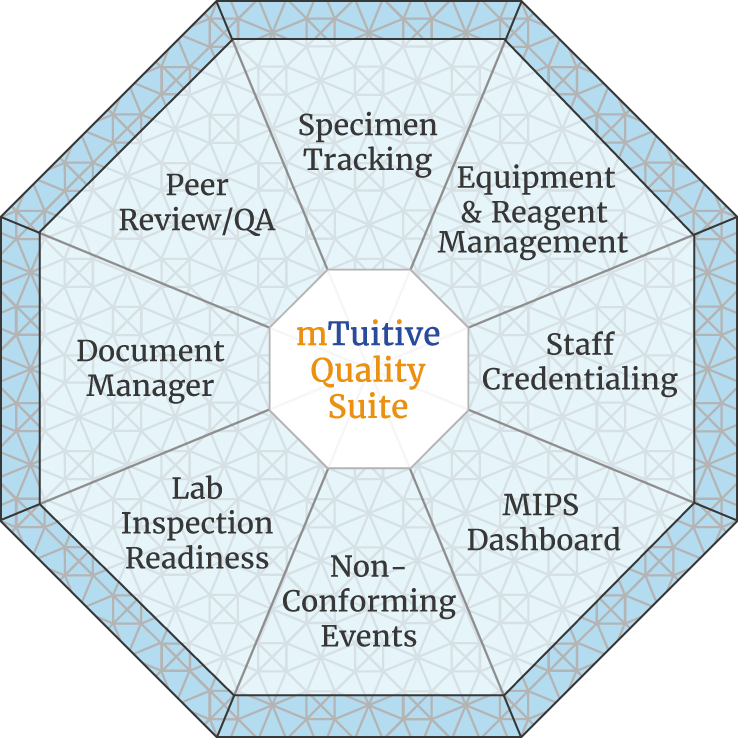
mTuitive's Quality Suite offers multiple modules for pathology labs looking to monitor, quantify, and improve the quality of their practices in the lab.
There are multiple offerings that either work together or can be obtained independently that empower labs to have better data about their workflows and consolidate their processes in one location. Improve quality, increase productivity, and reduce costs through the mTuitive Quality Suite.
Ensure that your lab is ready for inspection, all of your practices follow best of breed standards, and that you and your staff are performing to the best of your abilities with a seamless workflow.
mTuitive has incorporated best—of—breed recommendations from multiple government and organizational authorities in assembling these products. These include CLIA 1988 Guidelines, CAP QA Guidelines, Joint Commission, Association of Directors of Anatomic and Surgical Pathology, QAAP recommendations Pan-Canadian Conference on Interpretive Pathology, and more.
Every module in the mTuitive Quality Suite is based on the core components of creating, instituting, and implementing quality assurance in laboratories. Those standards are Analysis, Documentation, and Corrective Action. Every module allows users to analyze the situation, properly document what transpired, and then recommend a course of action that will prevent problems and ensure best results.
mTuitive's Document Manager and Lab Inspection Readiness reduces time spent managing documents and policies, improving productivity by making content easier to find and proactively manages compliance e.g. TJC, ISO/CAP‐15189. It reduces risk by enabling effective governance of healthcare information; at the same time ensures that information accessed through a central knowledge base is current and approved.
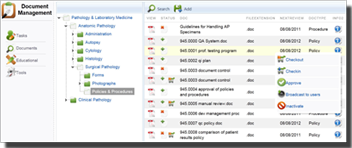
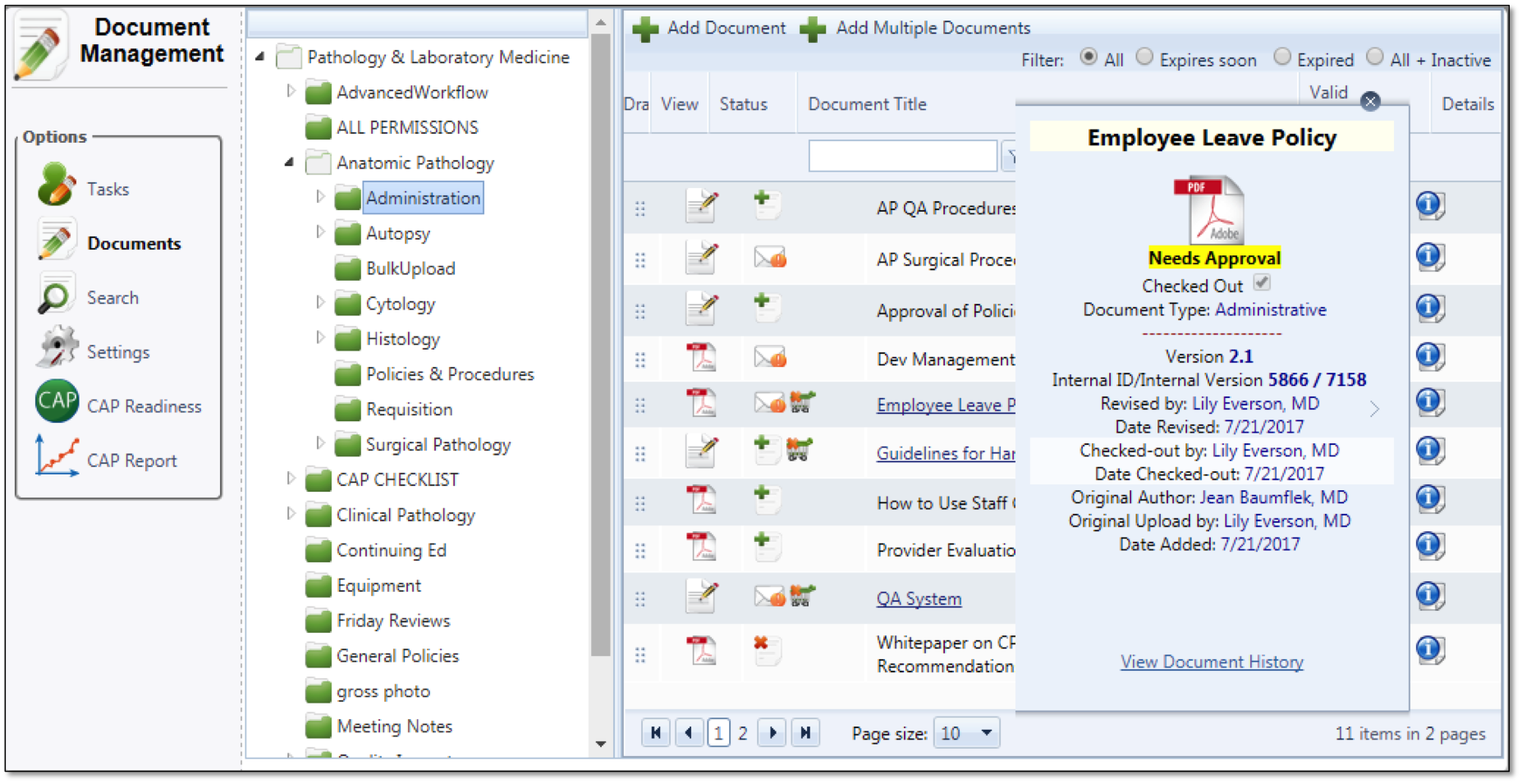
Manages electronic documents in most file formats. Organizes documents into hierarchies of folders that reflect the different ways in which people work. A single click configures the rules of the folder. You can easily set different levels of permissions. This helps you fine tune the type of access that you want to grant to individuals and groups based on your department or institution's policies.
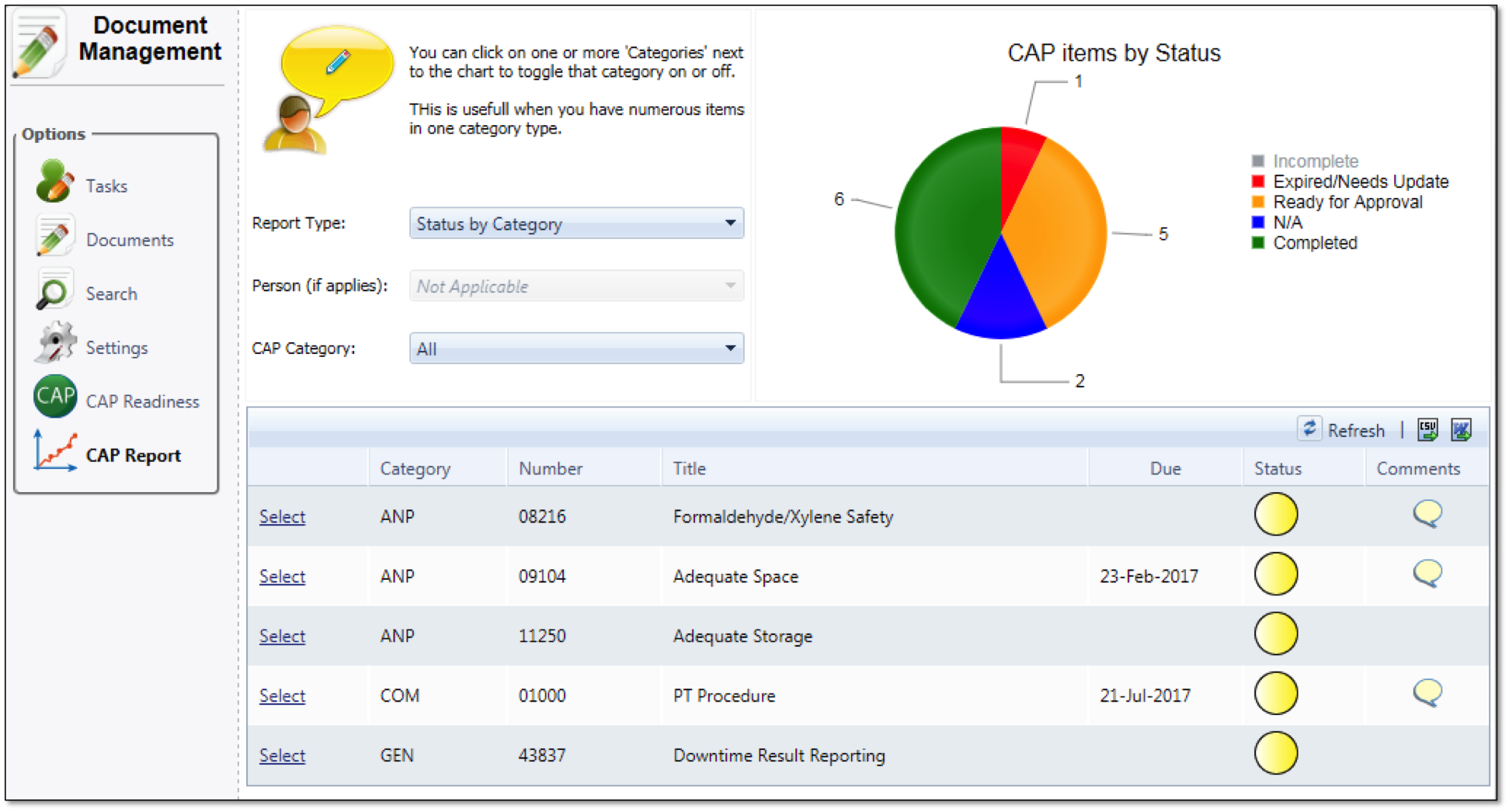
Know with a glance if you are in compliance with your current checklist. Status indicator lights allow you to manage proactively. Simply clicking on the element provides you the details you need to manage to your checklist requirements. Both checklist component items and changes to checklist are configuration controlled for ease of management. Apply custom data to documents: Lets you associate extra information with documents. Metadata is indexed and can be used to easily retrieve and generate reports on documents based on your custom criteria.
Automate document management processes, such as document change requests, document review and approval processes to ensure that they are carried out accurately and consistently according to your own requirements or according to those imposed by regulatory agencies.
This helps insure that all printed documents have the required information to insure the document is authorized, approved and the correct version. It also includes a next review or obsolete date as necessary. Our PDF robot places the indicia with the elements you've defined.
Supports CAP, The Joint Commission, CLIA and Provincial compliance along with Six Sigma quality initiatives.
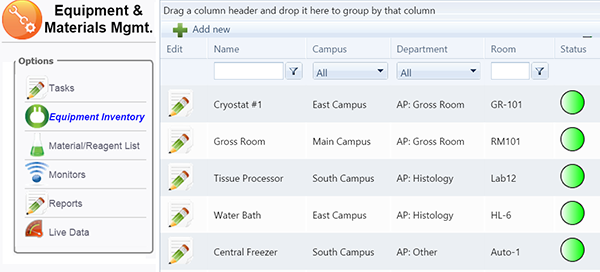
The monitoring of temperature sensitive areas and the proper maintenance of equipment is critical for proper laboratory operation. Even though all labs strive to employ the best staff, the use of automated monitoring ensures the integrity of specimens, reagents, media and equipment used throughout the lab, while ensuring compliance.
Lights‐Out operation
Provisions for Wi‐Fi wireless communication and wired LAN
Automatic assignment of alerts / tasks to groups or individuals
Promotion of alerts / tasks for approvals for deviations with automatic resolution documentation
User defined ranges, schedule, history, deficiency resolution
User configurable buffers / schedules to minimize false alarms
Reduces inventory loss due to equipment failure
Conformance for CAP, AABB, CLSI, OSHA, DOT, CLIA, AAALAC, FDA, GAMP, GxP, HACCP, Joint Commission, USP 797, Provincial or other criteria with associated inspection reports
Predefined equipment, reagent and material templates
Supports both scheduled and unscheduled events
Certification validation of users as necessary, e.g. calibration
Monitoring for expirations and handling for compliance
Tasks can include instruction manuals, MSDS and videos
Creates log files to CAP, TJC, CLIA and other standards
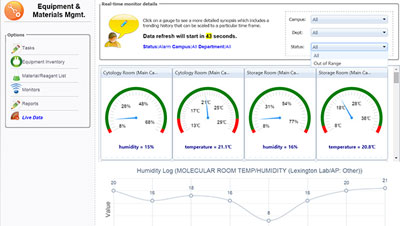
Working with your LIS to automatically identify, assigning cases, gathering meaningful details, improving quality while maintaining compliance with OPPE / FPPE reviews. Dramatically enhance your ability to provide accurate and timely diagnoses in support of clinical decisions, reducing risks while automating most of your QA and inspection reports.
Monitors pre‐analytic, analytic and post‐analytic variables that contribute to diagnostic errors
Triggers automatic peer‐review identifying potential QA to be performed from your LIS
Provides reports necessary of ongoing process improvement and CAP / TJC Inspections
Full pathology staff management features reduce administrative overhead
Automates the OPPE and FPPE mandated processes leveraging excluded, vetted and weighted data
Reduces time performing / analyzing QA—Minutes/day, can work within your LIS environment
Increases QA metrics / details—Helpful data to improve patient safety and identify bottlenecks
Automates the process—Guides your staff through QA and Peer Review
Eliminates painful QA analysis crunch—No need to submit periodic QA analysis or inspection data
Integrates with your LIS—No redundant data entry
Central QA repository—One location for all QA reporting, dashboards and alerts
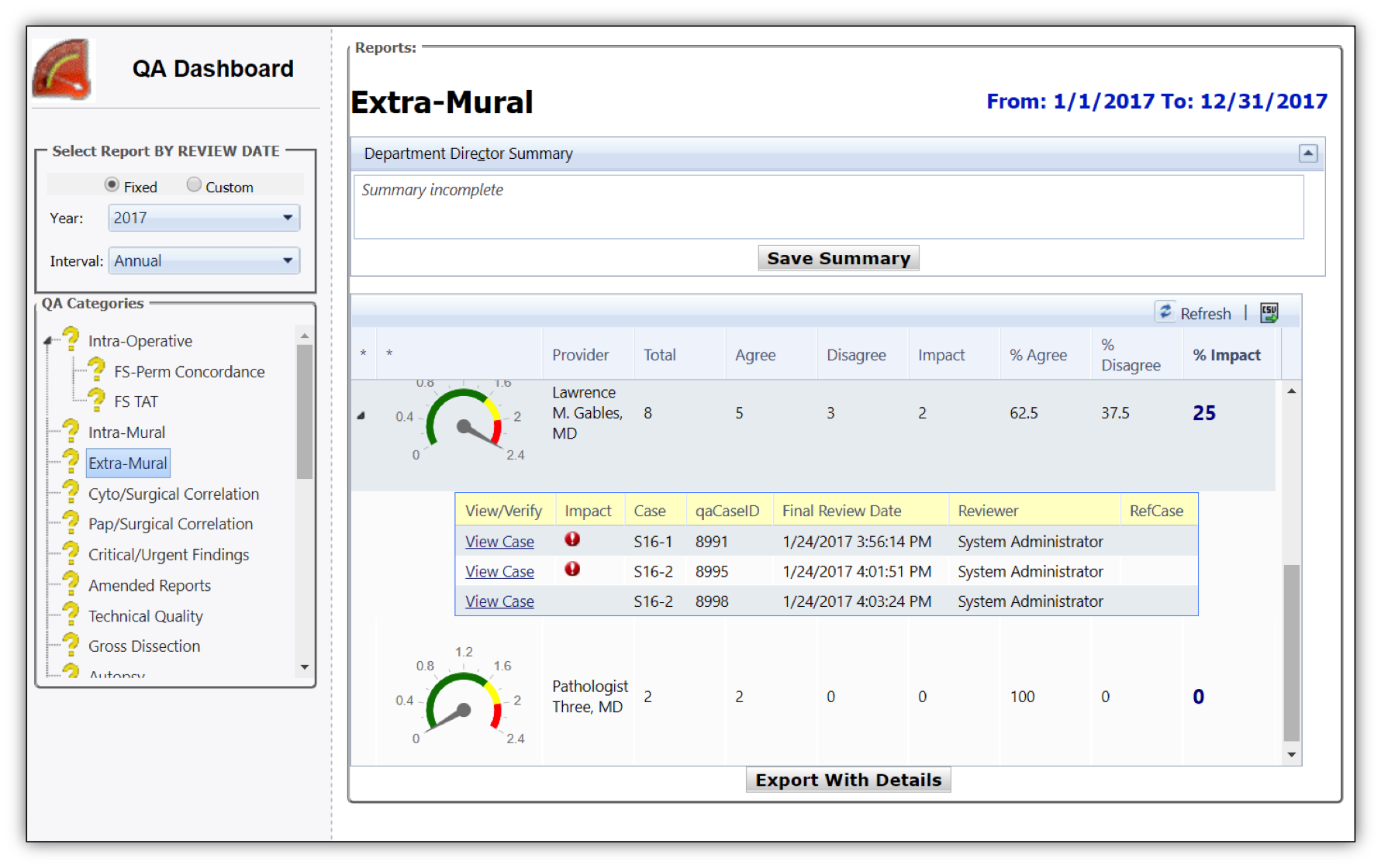
Intraoperative Cases (e.g. Frozen ‐ permanent section concordance and TAT)
Intradepartmental reviews (e.g. First time diagnoses of malignancy)
Extramural (Inter‐institutional) reviews
Cyto‐Surgical Pathology Correlation (e.g. Concordance of FNA to surgical specimen)
Technical and Gross dissection quality of histologic and cytologic preparation
Amended report review e.g. Amended / appended cases
TAT (e.g. By part type with automated exclusions)
Autopsy (e.g. Report adequacy and turnaround time)
Support for new staff and Residents
Advanced LIS Search for ad hoc analysis
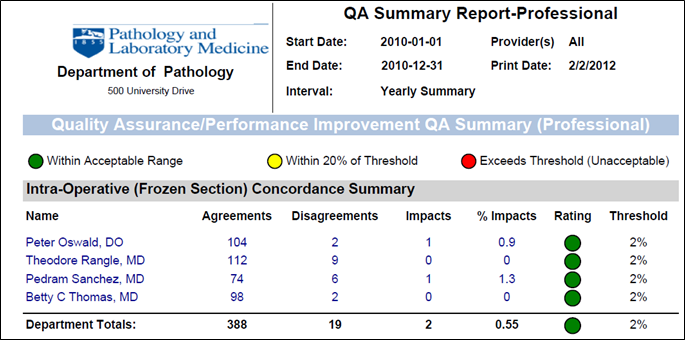
These default standards have been chosen based upon recommendations from CAP, TJC and can be adjusted:
Frozen / Permanent Section Correlation ‐ e.g. <3% discordance for cases associated with clinical significance to patient mgmt
Interdepartmental review of first time diagnosis of malignancy ‐ e.g. <2% discordance of cases associated with significance
Extramural ‐ e.g. <2% discordance for cases associated with clinical significance to patient management
Cyto‐surgical pathology correlation ‐ e.g. <2% discordance for cases associated with significance
Amended report review ‐ e.g. <2% discordance for cases associated with clinical significance
Report turnaround time (surgical pathology and cytopathology) ‐ e.g. 80% of cases signed out within 2 working days, 95% of cases signed out within 5 working days
Other user‐defined measures with self‐administered configuration and exclusions processing
Dashboard status of green, amber and red lights to highlight issues so you can manage‐by‐exception. Functional sorting of the staff issues that are important for your specific needs for proactive management. Includes over 80 parameters including:
Employee service type and licensing with location and authorized function matrix
Employment contract details, anniversary, renewals
Continuing education credits
Degrees and supporting documentation including diplomas, transcripts, certificates, etc.
Background, contact information
Review Dates: last, next with supervisor(s) process automation
Document attachments and general comments

Supports CAP, The Joint Commission, CLIA and Provincial compliance along with Six Sigma quality initiatives.
In order to maintain your staff's proper credentials, so they are approved to handle the tasks needed of them, mTuitive Staff Credentialing is a streamlined approach to keeping all staffing credential needs in one location. Put in tasks for when staff needs to get new credentials or renew current ones. Eliminate the anxiety of inspections and ensure personnel is up to code.
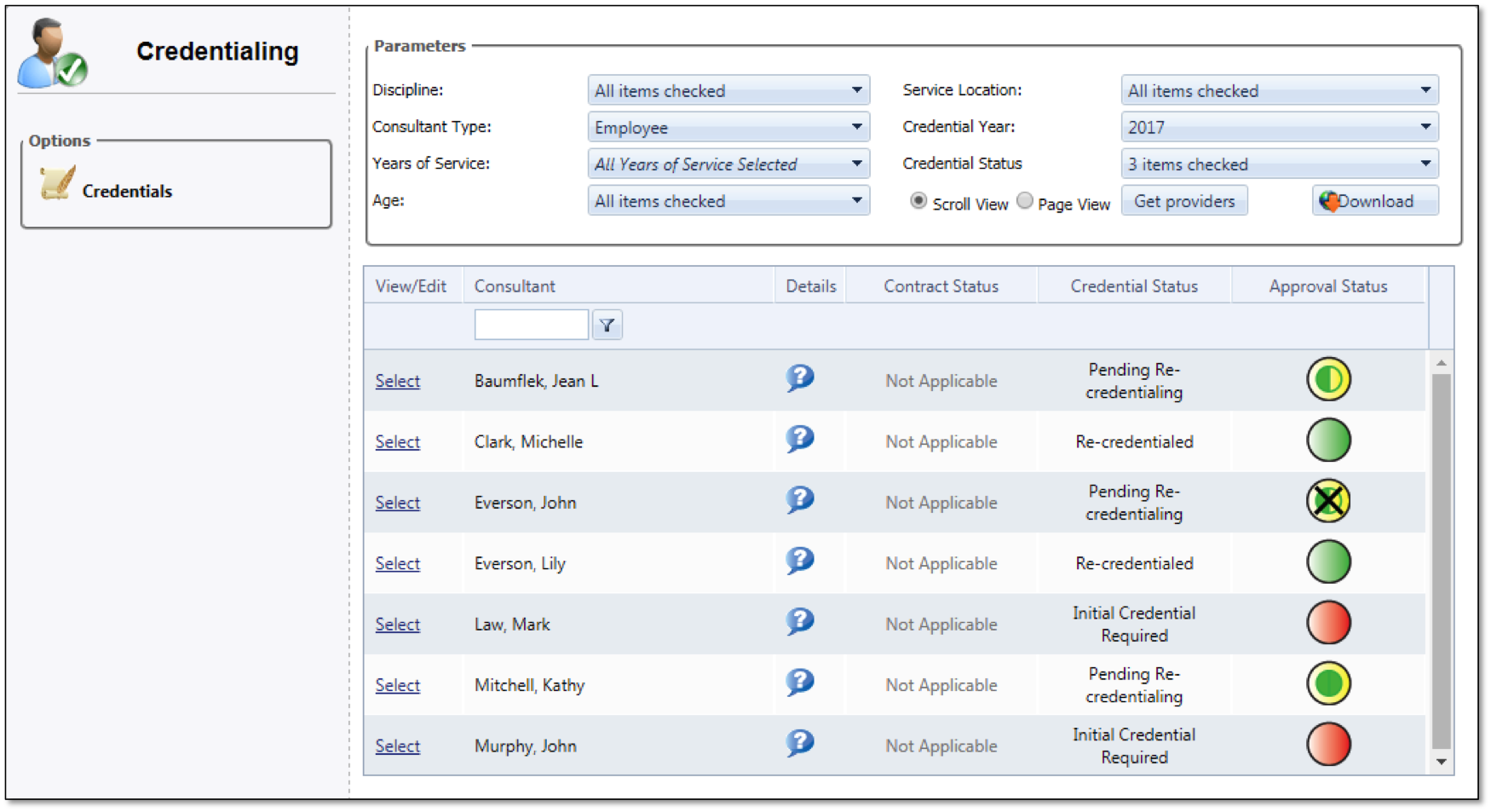
One location for all staff credentialing
Handles both technical and professional needs
Automated task lists ensure proper personnel seek and receive their necessary credentials
Ensures credentials are current and ready for all inspections and other needs
Improves processes by streamlining credential information and requirements
Eliminates costly manual processes in favor of automated efficiency
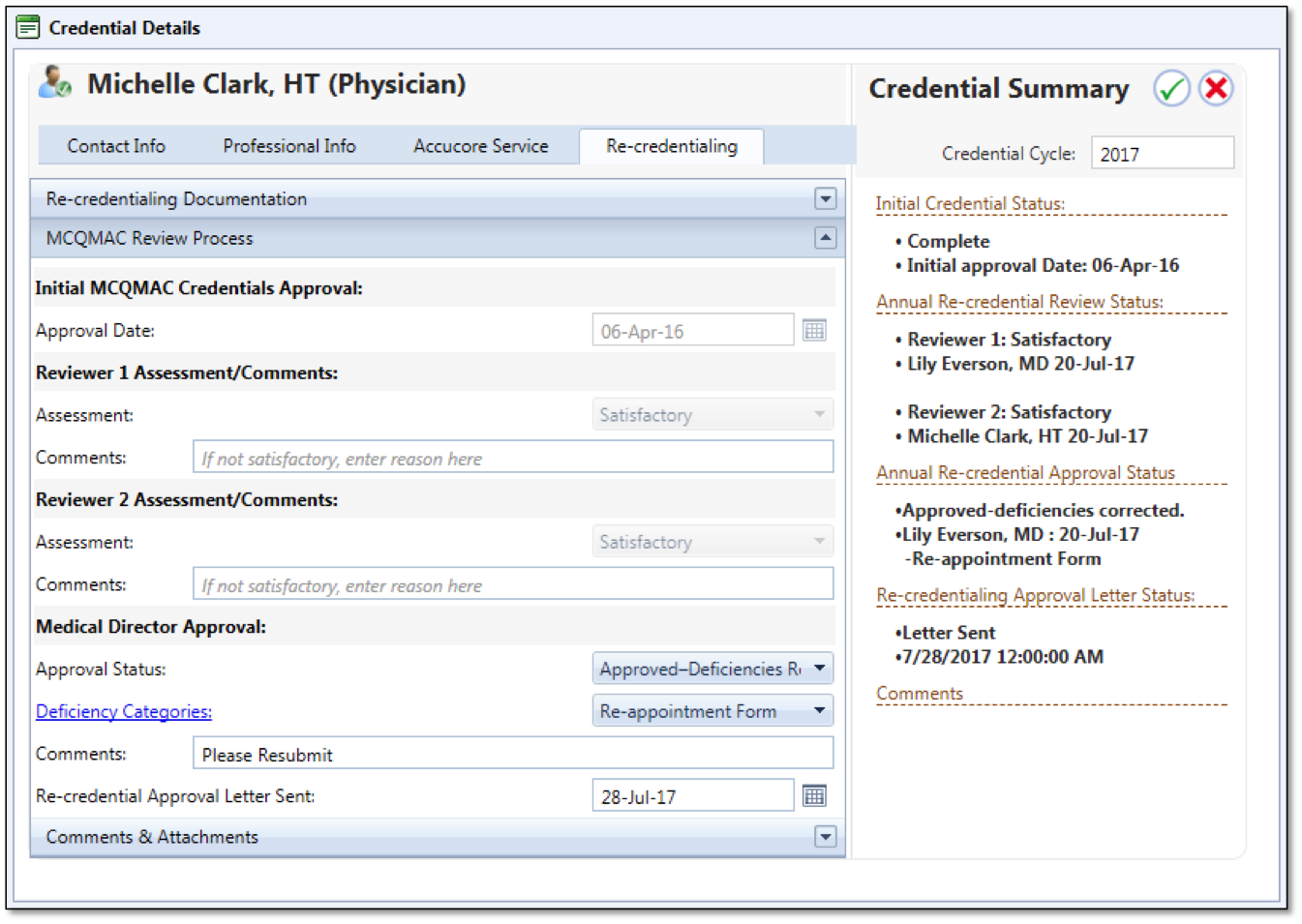
Supports CAP, The Joint Commission, CLIA and Provincial compliance along with Six Sigma quality initiatives.
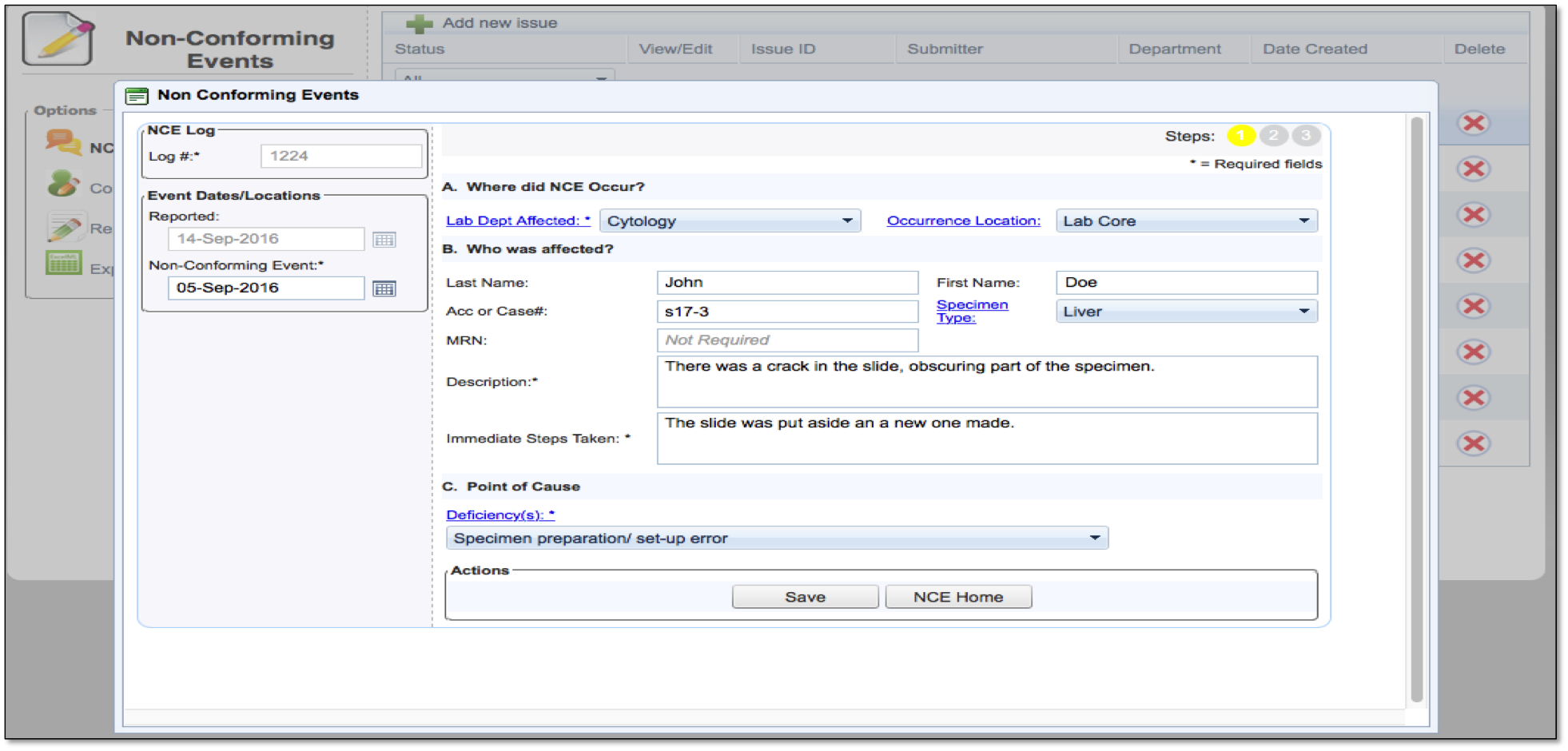
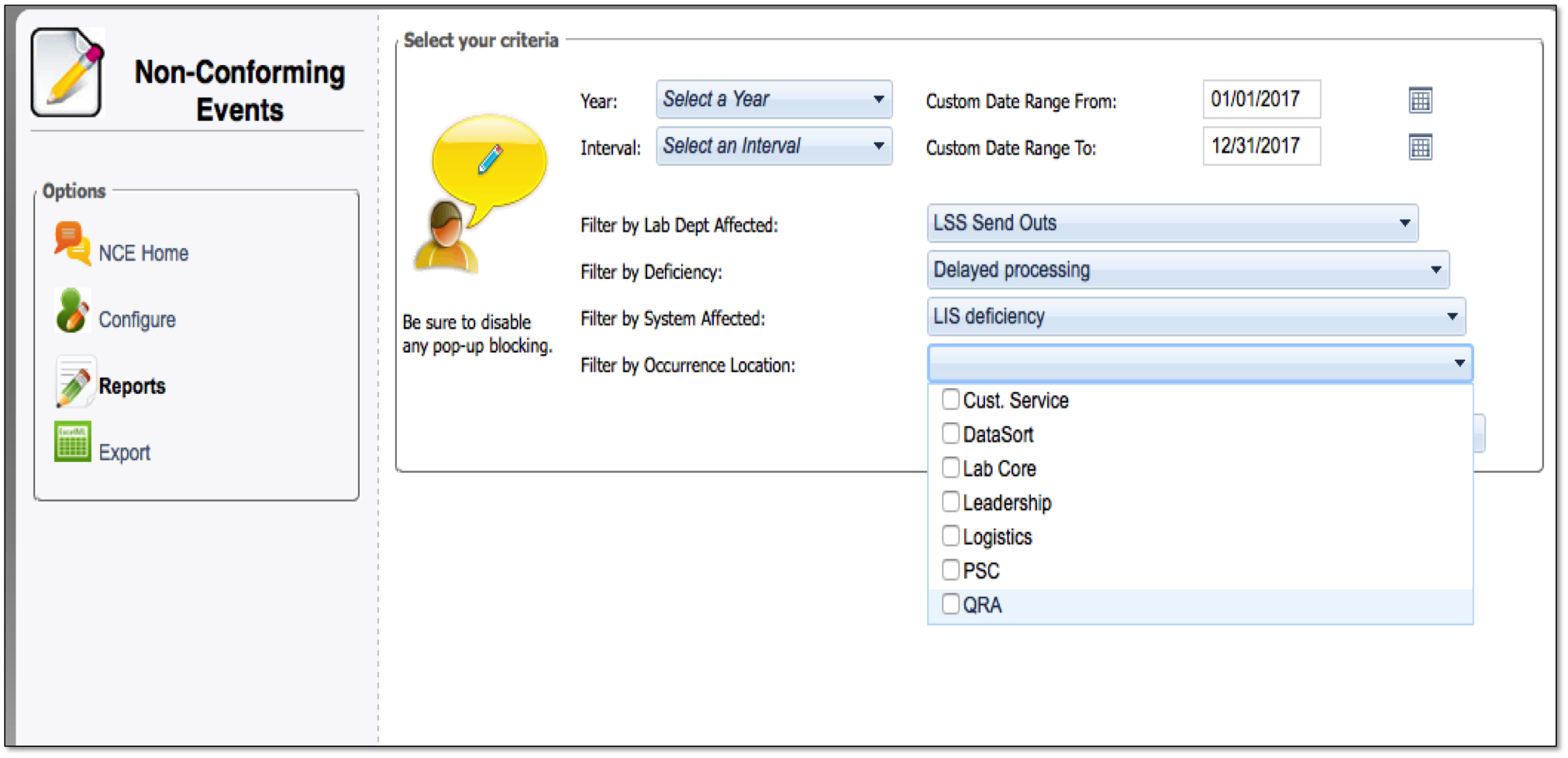
When NCEs do occur, labs need a clearly defined process for documenting them. This documentation allows lab professionals to analyze trends and figure out the best way to prevent them from ever happening again.
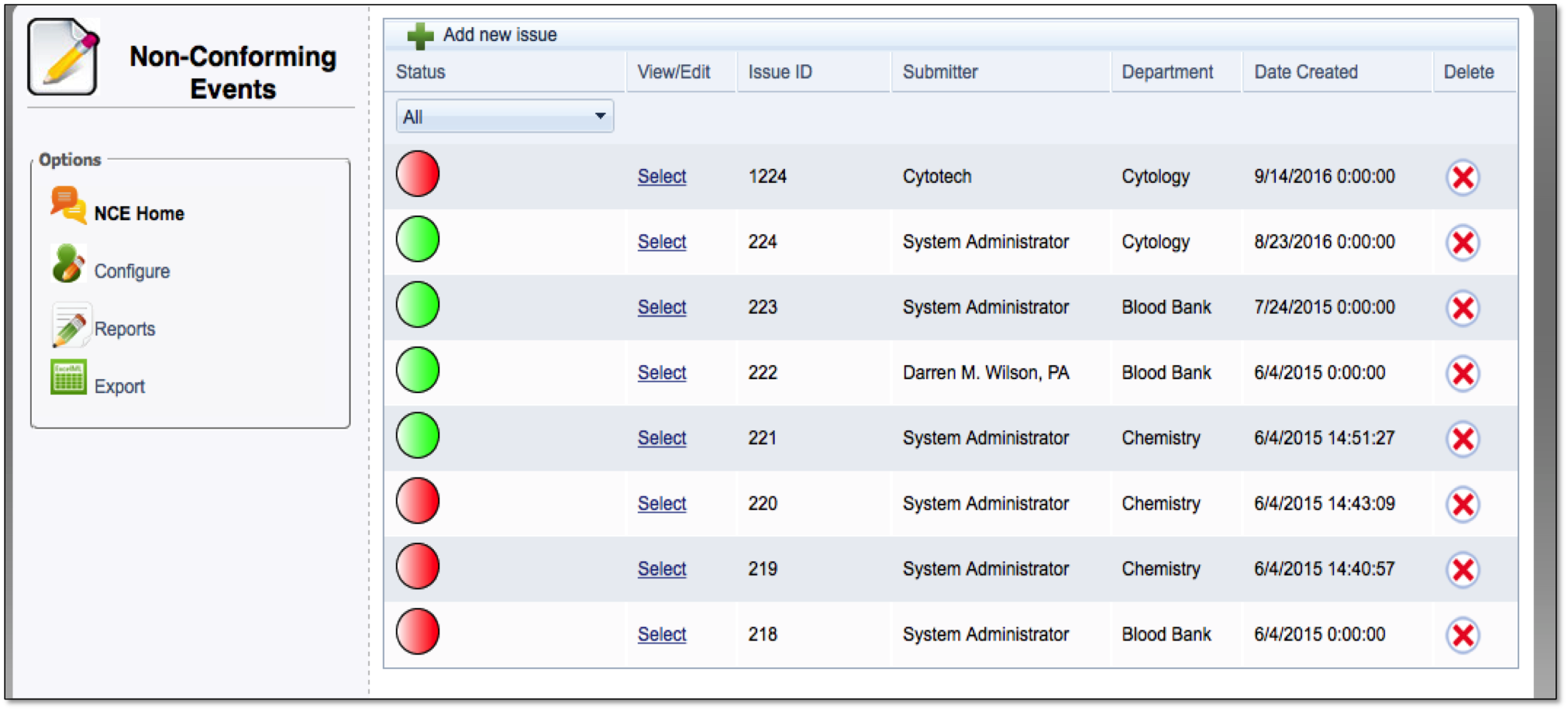
Centralized tracking of all Non-Conforming Events
Easy—to—read dashboard shows status of reports on NCEs
Details on how to avoid NCEs from repeating
Satisfies requirements for clear documentation for all NCEs
Increases QA metrics for your lab
Helps ensure that Non-Conforming Events don’t reoccur through documentation and analysis
Track specimens from accession to the lab with new AccuTrack AP and know the current status of any specimen inside of your lab. AccuTrack AP works the way you want without disrupting workflow. mTuitive's specimen tracking software helps pathology staff to address specific risk areas, integrate with and auto-print from the LIS, and ensure timely and efficient tracking of sensitive materials.
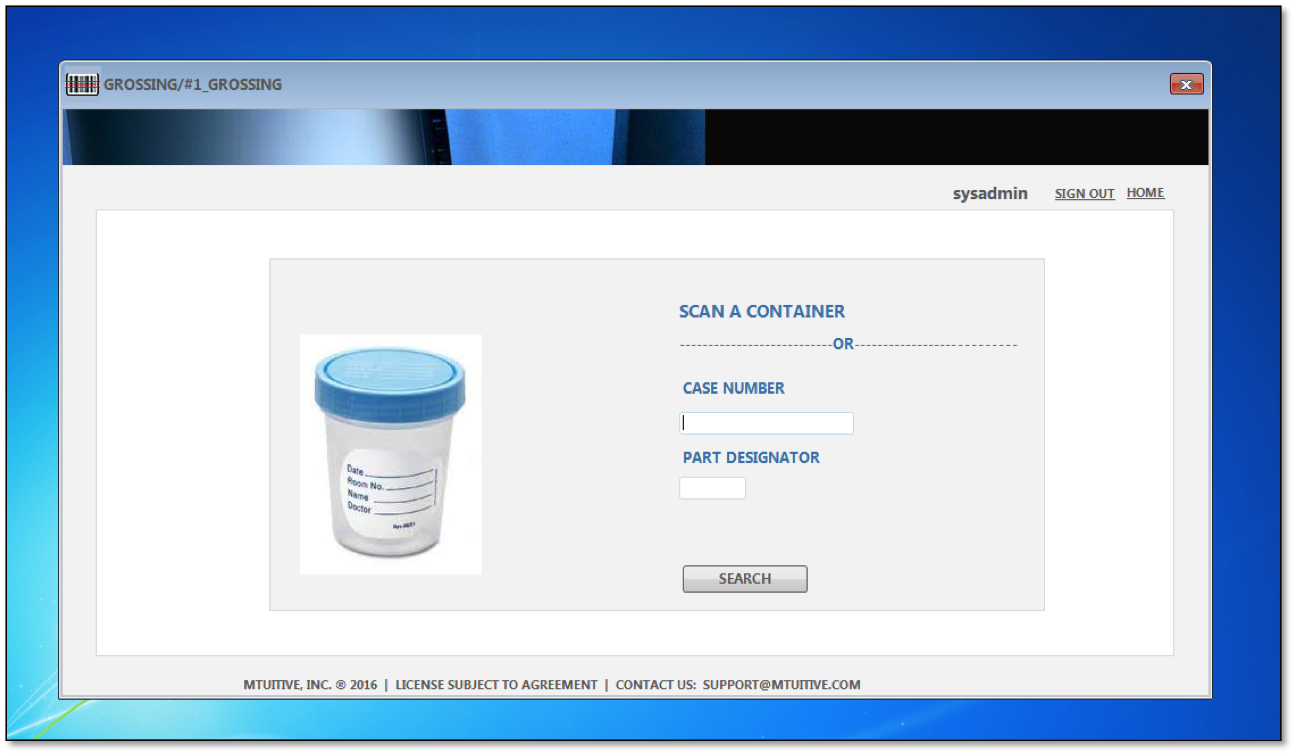
Eliminates error prone, redundant, and costly manual processes
Enjoy confidence of knowing where specimens are in your lab at all times
Comment on specimens and have a centralized location for all specimen tracking
Print out labels for slides and cassettes for efficient tracking
Utilizes multiple workstations for easy access
Monitors specimens from accessioning to diagnosis
There are a lot of changes happening to how pathologists are graded, monitored, and reimbursed. With all of the changes surrounding the Medicare Access and CHIP Reauthorization Act ( MACRA) and the Merit-based Incentive Payment System ( MIPS) underneath it, it's important that labs are able to easily track how they are doing with quality assurance.
Maintain and monitor your lab’s MIPS data for MACRA. Ensure that all physicians are submitting the necessary information to qualify for MACRA requirements and reimbursement while utilizing CAP eCC data to its fullest potential.
Centralized location for information on physicians’ reporting
Eliminate costs and time spent on assembling MIPS data for automatic entry
Maintain compliance with standards of reporting and identify areas of improvement
Automate abstraction to a QCDR for reporting to CMS
At—A—Glance Dashboard provides easy to read graphic details for each pathologist
Ensure proper reimbursement for staff based on the reporting and work they do.
