The system provides expert guidance and error checks based on standard, best-of-breed or homegrown protocols. Powered by mTuitive's proprietary expert system software, CAP eFRM/xPert for Pathology has significant advantages over other reporting options.
CAP eFRM/xPert for Pathology has templates available for Cancer Diagnosis, Gross Pathology and all Final Diagnoses. These templates currently cover over 95% of all potential surgical pathology cases, with more being created every month.
mTuitive helps your facility meet NHS COSD requirements. Learn more here.
For more information about your specific system, please contact us today.
Pathology departments are ensured compliance with in‐house and/or national standards — such as the new synoptic reporting requirements set forth by the Commission on Cancer. mTuitive follows all new standards development and, as part of our standard agreement, ensures your department maintains compliance.
CAP eFRM/xPert for Pathology provides a true single step sign out by capturing structured data in addition to case narratives at the point of examination. Results are automatically delivered to the pathology system which greatly reduces costs and errors associated with transcription.
Automated calculations and error checks eliminate simple human errors.
The process makes for accurate collection and comparison of cancer data which directly impacts cancer screening and treatment protocols.
Consistently offers an interpreting clinician quick, complete and concise documentation in a uniformed format. CAP eFRM/xPert for Pathology uses medically consistent terminology which improves communication and decreases questions from clinicians and cancer registrars. Customers of the pathology report are more satisfied by the reduction in report turnaround time.
Offer data to an interpreting clinician in an easy‐to‐read format with medically consistent terminology.
Automatically deliver data to software applications and Cancer Registry. Adds the ability to mine data across all pathology reports.
Provide time saving assistance and error prevention when diagnosing slides.
mTuitive templates enable institutions to follow established reporting standards from such professional associations as the College of American Pathologists, Cancer Care Ontario and the Royal College of Pathologists and the Cancer Outcomes and Services Dataset (COSD) for the UK.
Each set of templates is a point-of-departure for an institution's implementation — modifications will be made to suit the needs of your institution. mTuitive Knowledge Engineers, clients and partners are constantly developing new templates for synoptic reporting in order to meet your own institution's needs and requirements.
The Central Data Repository is mTuitive's dynamic and safe data repository that stores information entered into CAP eFRM/xPert for future reporting, data mining and/or abstraction.
Cancer Centers who maintain accreditation from the American College of Surgeons Commission on Cancer (ACS CoC) are required to meet minimum data reporting standards for the classification of malignant tumors. The College of American Pathologists (CAP) developed cancer protocols and checklists to assist pathology and oncology departments in meeting these standards. SNOMED® International, a Division of the CAP, developed the SNOMED CT encoded versions of these checklists. mTuitive, Inc. was the first company to license the SNOMED CT Encoded CAP Cancer Checklists and provides a fully electronic system for synoptic reporting of tumors for all of the checklists. The unique design of the product reduces (or, in many cases, eliminates) the report transcription and data abstraction associated with the cancer registry process.
In August 2005, mTuitive became the first company to execute a licensing agreement with The Royal College of Pathologists (London, U.K.) to create an electronic version of cancer reporting datasets developed by the Royal College to standardize the classification and reporting of malignant tumors. The datasets ensure consistency in the application of the standards, which will become required for the reporting of all malignant tumor cases in the United Kingdom. The capture of standardized reporting data will facilitate the automation of cancer registry reporting and data abstraction processes across the British healthcare system.
Many institutions have established their own reporting protocols analogous to those of CAP and RCP. Using mTuitive's Agile Author, institutions can easily develop their own protocols to meet the internal standards they have set forth. This gives users the freedom to create more advanced calculations, include specific data elements or organize their reports more to their liking.
View a demonstration of how easily xPert for Pathology integrates with Sunquest CoPath.
Contact us today to speak with pathologists from around the world who are using CAP eFRM/xPert for Pathology ‐ Cancer Reporting to meet their reporting requirements.
In mTuitive's quest to provide flexibility to pathologists, the xPert for Gross Pathology solution was created to provide gross (or macroscopic) pathologists with a product that has the same easy‐to‐use functionality and financial savings of our other products.
In addition to the ease of xPert for Pathology's true single step sign out, xPert for Gross Pathology captures structured data (in addition to case narratives) at the point of examination. Pathologists receive the gross description in a clear, concise format in a completely automated fashion. Our research has shown that pathologists and their customers are more satisfied by the xPert generated report and its reduced turnaround time as compared to those created through narration and transcription.
In collaboration with Massachusetts General Hospital, the mTuitive Human Factors Division created a completely hands-free, speech recognition driven user interface for structured data capture by pathology assistants and gross technicians while they conduct the gross evaluation of specimens.
Powered by the Dragon NaturallySpeaking™ speech recognition engine, the product allows the gross description to be to directly entered into the pathology report, thereby eliminating the need for expensive transcription services and removing the lag time in the reporting process caused by transcribe‐review‐edit cycles. The system also standardizes how all pathologists report their findings ‐ ensuring complete and accurate pathology reports.
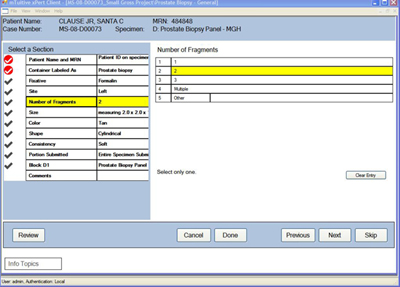
Professional associations have established requirements for cancer reports, but the standards for non‐cancerous pathology reporting are less defined. mTuitive works diligently to ensure that non-cancerous reports adhere to the specific standards of your institution.
mTuitive Knowledge Engineers, clients and partners are constantly developing new templates for synoptic reporting. With a projected 85% of all pathology reports dealing with non-cancerous cases, the addition of these templates to the xPert for Pathology solution extends all of the benefits (including the financial savings) to the majority of the pathologist's workflow.
The standard offering for xPert for Pathology uses the xPert Client User Interface. Designed by mTuitive’s Human Factors division and tested with practicing pathologists, xPert Client is maximized to capture data quickly and efficiently while delivering appropriate expertise and decision assistance.
Knowledge engineers are experts at constructing meaningful, useful and simplistic protocols. mTuitive trains members of your pathology department to become knowledge engineers using the Agile Author. Knowledge engineers break down the information passed on by their pathologists to create standard templates that comply with department reporting measures.
New templates are created every month by our community of Knowledge Engineers. When a new template is ready for use, it is placed in our online community where it can be shared with the rest of the Agile Author clients. Using our discussion forums, Agile Author users can share ideas and collaborate on development of templates for new standards. Advanced training and helpful tips are available as well.